Continuing with the understanding
of radiocarbon dating (Carbon-14), and the time clock Willard F. Libby (left)
invented to read the ages of the past used constantly by archaeologists and
anthropologists in determining the age of past civilizations.
Once again, to
understand how the radiocarbon dating time clock works, and its affect on our undertanding of the age of various ruins found throughout the Americas, cosmic rays enter the
earth's atmosphere in large numbers every day and when one collides with an
atom in the atmosphere, it can create a secondary cosmic ray in the form of an
energetic neutron. When these energetic neutrons collide with a nitrogen-14
(seven protons, seven neutrons) atom it turns into a carbon-14 atom (six
protons, eight neutrons) and a hydrogen atom (one proton, zero neutrons). Since
Nitrogen gas makes up about 78 percent of the Earth's air, by volume, a
considerable amount of Carbon-14 is produced. The carbon-14 atoms combine with
oxygen to form carbon dioxide, which plants absorb naturally and incorporate
into plant fibers by photosynthesis. Animals and people take in carbon-14 by
eating the plants and/or the animals.
Red Arrow: When cosmic rays bombard the
earth’s atmosphere, they produce neutrons. Green Arrow: These excited neutrons
then collide with nitrogen atoms in the atmosphere, changing them into
radioactive carbon-14 atoms; Blue Arrows: Plants absorb this carbon-14 during
photosynthesis. When animals eat the plants, the carbon-14 enters their bodies.
The carbon-14 in their bodies breaks down to nitrogen-14 and escapes at the
same rate as new carbon-14 is added. So the level of carbon-14 remains stable; Yellow
Arrow: When an animal dies the carbon-14 continues to break down to nitrogen-14
and escapes, while no new carbon-14 is added. By comparing the surviving amount
of carbon-14 to the original amount, scientists can calculate how long ago the
animal died
It is also
interesting to note that between 1945 and 1947, Libby decided to pursue the
radiocarbon dating project in secret, though he broadcast the subject of his
ultimate goal during a Christmas party in 1946, which astounded one of his
colleagues, James Arnold, who had
been working under Libby’s direction isolating the first millicurie of reactor-produced
Carbon-14.
It should also be noted that at this time Carbon-14 had not yet been
isolated in nature, yet Libby’s now announced plan was to date archaeological
history with an isotope of carbon. However, when his pre-mature announcement
was given, it upset the archaeological world who wanted to know why the
physicist was getting involved in archaeology and trying to tell them how to
date their finds. By his own admission, Libby stated: “I have no competence in
the field of archaeology.” This led to a two-year series of diverse
encounters between the physicists and the humanists (the latter being the
American archaeologist’s intellectual
orientation). That is, it described the problems in cross-discipline
communication, i.e., communicating to archaeologists the concepts of chemistry
and physics involved with Carbon-14.
So, for the next two years, from 1947 to 1949, Libby, Anderson and Arnold
worked more closely with archaeologists to help them better understand the
dating process Libby wanted to create and how that would benefit archaeology. This
involved the creation of the “Supper Conferences,” a dinner held every two
weeks by the Viking Fund, a supplier of funds for risk-involvement research.
These dinners drew anthropologists on the Eastern Seaboard to attend cocktails
and dinner for informal discussions—an innovative way for conveying novel ideas across
disciplines in an informal mode of conversation.
It was explained that once equilibrium in the atmosphere reached
equilibrium (about 40,000 to 60,000 years after creation (however, after 50,000
years, there is so little Carbon-14 left in any specimen that it is very hard,
almost impossible, to calculate its age beyond that point, so a figure of
50,000 years is usually given as the end date), then no more carbon would enter
the system after death. That, then, would allow the scientist to determine how
old it was at death by measuring the half-life cycle (the standard way of
expressing the decay rate was called “the half-life”), so in knowing how much
Carbon-14 element was left in the artifact, it could be determined how many
“half-life” cycles had passed and date the artifact within a reasonable time
frame—say, to 500 B.C., plus or minus 150 years (meaning the artifact died
between 650 B.C. and 350 B.C.
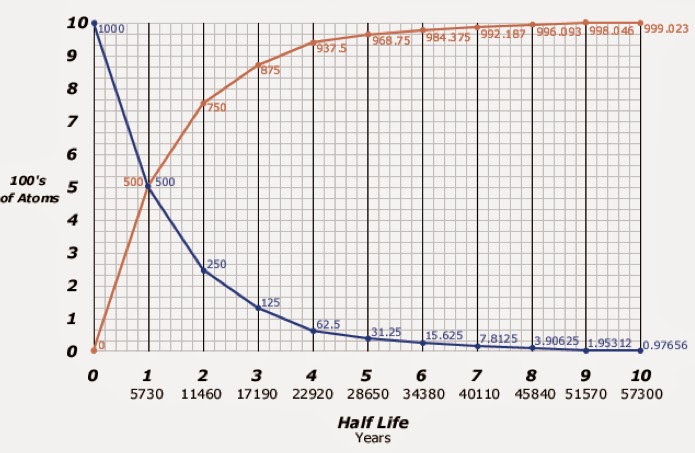
Looking at the blue line only, we can see how many of the
100 Carbon-14 atoms remain during 10 half-life cycles ending at 57,300 years.
As an example, 75% of Carbon-14 is gone after two half-life cycles, or 11,460
years
It was also explained that this dating was possible
since after plants
and animals perish, they no longer replace molecules damaged by radiocarbon
decay. Instead, the radiocarbon atoms in their bodies slowly decay away, so the
ratio of carbon-14 atoms to regular carbon atoms will steadily decrease over
time. This is based, of course, on the Earth having achieved equilibrium, i.e.,
being at least 50,000 years old.
But what if it was not that old? What
if during its life, a plant or animal was not yet exchanging carbon with its
surroundings and its carbon did not have the same proportion of Carbobn-14 as
the bioshphere and the carbon exchange reservoir? What if there was still
Carbon-14 entering the atmosphere and building up? How then could the clock be
set to equilibrium?
The Carbon Exchange Reservoir. It is based on the belief that the Earth
is older than 50,000 years and that equilibrium of Carbon-14 had already built
up in the atmosphere
But if Carbon-14 had not reached
Equilibrium in the atmosphere, that would mean that the Earth was less than 50,000 years old!
To test his theory and the
“clock” he had developed, Libby took several Egyptian artifacts that were
already datable by other techniques and tested them. A
committee of advisers consisting of Donald Collier, Richard Foster Flint,
Frederick Johnson, and Froelich Rainey was appointed to select the samples for
use and to help collect them. These distinguished gentlemen worked hard for
several years, assisting and collecting the samples and advising Libby and his
team. The research in the development of the dating technique consisted of two
stages—the historical and the prehistorical epochs. As Libby said in his 1960
Nobel Lecture: “The first shock Dr. Arnold and I had was when our advisers informed
us that history extended back only to 5,000 years. We had thought initially
that we would be able to get samples all along the curve back to 30,000 years,
put the points in, and then our work would be finished.”
Libby knew that in reading statements
in books that such and such a society or archeological site was 20,000 years old,
he was surprised to learn that these ancient dates were not really known dates,
but numbers the archeologists came up with. “In fact,” Libby added , “it is at
about the time of the First Dynasty in Egypt [3200 B.C.] that the first historical date of
any real certainty has been established. So we had, in the initial stages, the
opportunity to check against knowns, principally Egyptian artifacts, and in the
second stage we had to go into the great wilderness of prehistory to see
whether there were elements of internal consistency which
would lead one to believe that the method was sound or not.”
What he found was even more of a
shock!
(See the next post, “How
Old is Old? – Part III,” to see how and why Libby’s clock was set to read the
wrong time for radiocarbon dating and what impact that has on our understanding
the past and the age of the Earth)
Subscribe to:
Post Comments (Atom)







No comments:
Post a Comment